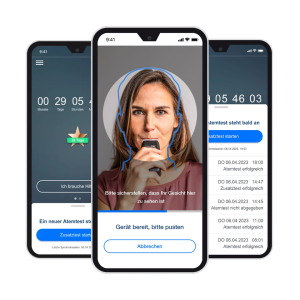
Our products
BAYOOCARE: We place medical products on the market in accordance with ISO 13485 and IEC 62304. Our services range from Regulatory Affairs and quality assurance measures to post-market surveillance. In doing so, we consistently draw on the expertise of the BAYOOMED business unit and its extensive experience with medical device classes I, IIa, IIb, and III as well as software safety classes A, B, and C.
These products have already been successfully placed on the market: