 https://www.bayoocare.com/wp-content/uploads/sites/10/2022/10/MDR-Zertifikat_Canva-v2.png
820
1200
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
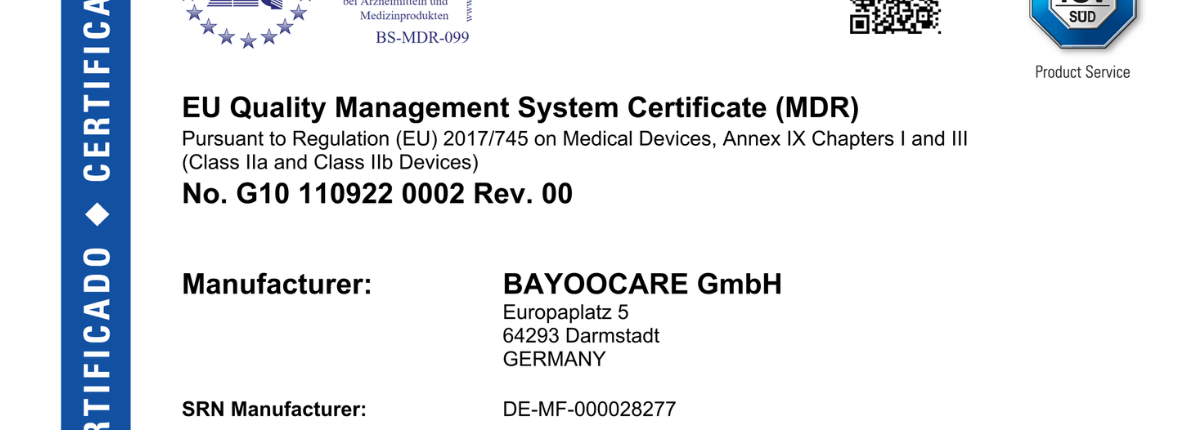
Julia Pfeffinger2022-10-20 14:08:322022-10-20 14:08:32MDR Certificate for Class IIb Medical Device
https://www.bayoocare.com/wp-content/uploads/sites/10/2022/10/MDR-Zertifikat_Canva-v2.png
820
1200
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-10-20 14:08:322022-10-20 14:08:32MDR Certificate for Class IIb Medical Device https://www.bayoocare.com/wp-content/uploads/sites/10/2022/10/MDR-Zertifikat_Canva-v2.png
820
1200
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-10-20 14:08:322022-10-20 14:08:32MDR Certificate for Class IIb Medical Device
https://www.bayoocare.com/wp-content/uploads/sites/10/2022/10/MDR-Zertifikat_Canva-v2.png
820
1200
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-10-20 14:08:322022-10-20 14:08:32MDR Certificate for Class IIb Medical Device https://www.bayoocare.com/wp-content/uploads/sites/10/2022/09/shutterstock_360391553-scaled.jpg
1707
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-09-29 07:18:412022-09-29 07:18:41One audit, five markets: Opportunities of the Medical Device Single Audit Program (MDSAP)
https://www.bayoocare.com/wp-content/uploads/sites/10/2022/09/shutterstock_360391553-scaled.jpg
1707
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-09-29 07:18:412022-09-29 07:18:41One audit, five markets: Opportunities of the Medical Device Single Audit Program (MDSAP) https://www.bayoocare.com/wp-content/uploads/sites/10/2022/02/shutterstock_295184639-scaled.jpg
1707
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-02-25 13:12:512022-03-01 13:12:43Regulatory backlog: why medical device manufacturers should not delay MDR implementation
https://www.bayoocare.com/wp-content/uploads/sites/10/2022/02/shutterstock_295184639-scaled.jpg
1707
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-02-25 13:12:512022-03-01 13:12:43Regulatory backlog: why medical device manufacturers should not delay MDR implementation https://www.bayoocare.com/wp-content/uploads/sites/10/2022/02/shutterstock_164724209-scaled.jpg
1706
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-02-03 10:38:582022-02-03 12:40:44MDR and IVDR: differences and similarities of the regulations
https://www.bayoocare.com/wp-content/uploads/sites/10/2022/02/shutterstock_164724209-scaled.jpg
1706
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-02-03 10:38:582022-02-03 12:40:44MDR and IVDR: differences and similarities of the regulations https://www.bayoocare.com/wp-content/uploads/sites/10/2022/01/shutterstock_561931702-scaled.jpg
1581
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-01-20 12:49:302022-01-20 12:52:34Artificial intelligence: 4 questions you should have an answer to
https://www.bayoocare.com/wp-content/uploads/sites/10/2022/01/shutterstock_561931702-scaled.jpg
1581
2560
Julia Pfeffinger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Julia Pfeffinger2022-01-20 12:49:302022-01-20 12:52:34Artificial intelligence: 4 questions you should have an answer to
